Related Posts
-
 19 febrero, 2020
19 febrero, 2020ResMed ventilators recall is Class I
FDA today designated a ResMed (NYSE:RMD) recall of certain Stellar non-invasive and invasive ventilators as Class I — the agency’s most serious level. The sound alarm on the ventilators may not work if the device has a failed electronic part, is stored without AC power for more than 36 hours, or powers on automatically when connected …
-
 10 octubre, 2019
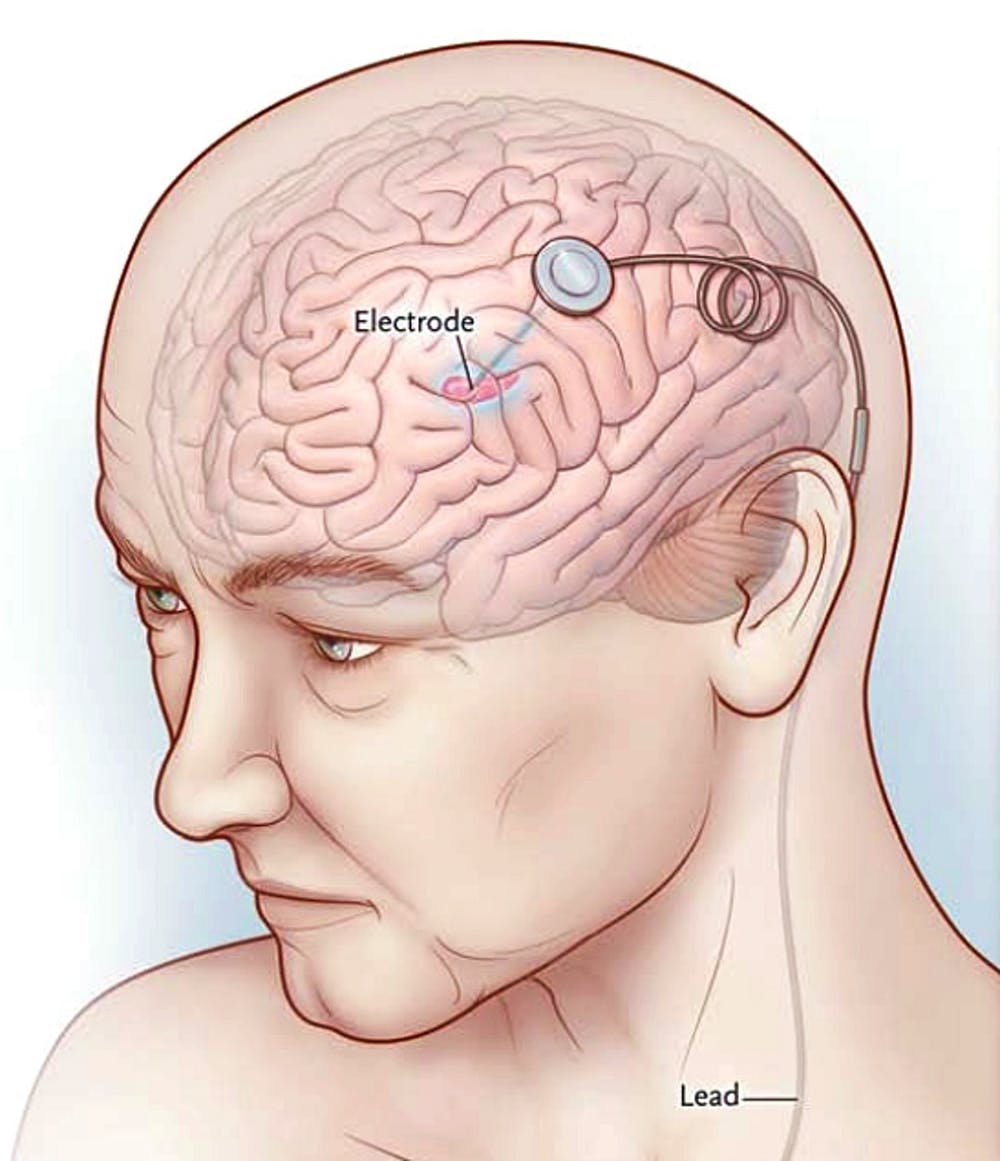
10 octubre, 2019Deep brain stimulation can be effective for severe depression
Researchers who monitored people with deep brain stimulation implants for 8 years suggest that the treatment can benefit those with severe depression. Regulators in the United States have already approved deep brain stimulation for the treatment of Parkinson’s disease, epilepsy, essential tremor, and obsessive-compulsive disorder. The treatment involves implanting wires into the brain and a stimulator in the chest …
-
 30 diciembre, 2019
30 diciembre, 2019Medical Devices; Exemptions From Premarket Notification for Class I and Class II Devices
«The Food and Drug Administration (FDA, Agency) identified a list of class I devices and class II devices that are now exempt from premarket notification requirements, subject to certain limitations. FDA published the lists of final determinations in accordance with procedures established by the 21stCentury Cures Act (Cures Act). Although each classification regulation for each …
-
 10 octubre, 2019
10 octubre, 2019Study: Abbott, Edwards may have mislabeled hundreds of patient deaths
Abbott (NYSE:ABT) and Edwards Lifesciences (NYSE:EW) may have mislabeled patient deaths as injuries or device malfunctions in hundreds of FDA adverse event reports involving their transcatheter aortic or mitral valve replacement devices, according to a new study. The study by JAMA Internal Medicine editor Dr. Rita Redberg, former FDA unique device identification (UDI) external program manager Madris Tomes and others examined a total …